This year is coming to it’s end, but not without sharing with you all the latest news of November!
🌟Drug development simulation
During the month of November 2025, our associated partner Learning by Simulation organized a practical online training for our DCs. The course is part of the RETORNA doctoral trainining programme foreseen for the selected 10 DCs.
This course was a hands-on simulation that showed PhD students how a new medicine moves from the lab to early human testing. It guided them through the big steps that happen after scientists discover a promising compound, such as:
- Checking safety first – making sure the compound isn’t toxic and behaves predictably.
- Producing reliable batches – learning why medicines must be made under strict quality rules so they’re pure and stable.
- Planning first human trials – understanding how early studies are designed to test safety and dosage before looking for signs that the drug works.
- Building a convincing case – learning how to show that a drug is safe, effective, and worth investing in for further development.
The course was taught as a team-based simulation, so participants worked together on realistic tasks, guided by experts.
By the end of the program, participants demonstrate the ability to integrate preclinical safety-driven selection, compliant batch supply, early clinical study design, and strategic Proof of Concept planning into a coherent development pathway. This ensures they are well prepared to contribute to the advancement of innovative therapeutics with both scientific rigor and translational foresight.
Learning Objectives
After completing and passing this simulation, participants will be able to:
- Recall the critical steps and regulatory requirements involved in advancing a drug candidate from lead optimization through phase 2 clinical development
- Explain the importance of nonclinical safety parameters and their role in candidate selection
- Describe the process of producing and supplying GLP and GMP batches
- Apply nonclinical data to design phase 1 first-in-human protocols
- Evaluate the efficacy, safety, and unique selling points of drug candidates to support decision-making
- Design a coherent development pathway that integrates nonclinical safety, CMC, clinical trials, and strategic planning
🌟Secondments
- After quite a few months away on the other side of the world, Chiara is back in Spain from the 1st of November. These were quite intense months of working, growing, learning and obtaining results, as you can read directly from Chiara’s words:
“💯 These almost six months in Denver on the beautiful campus of the University of Colorado University of Colorado Anschutz have been very intense.
🦸♀️ I have learned so much from a professional point of view, and being away from everyone and everything I knew and was familiar with for so long has given me the opportunity to better understand my limitations and strengths, which has given me great confidence in myself and my abilities.
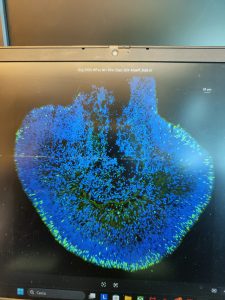
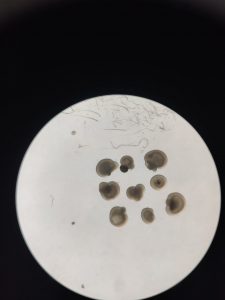
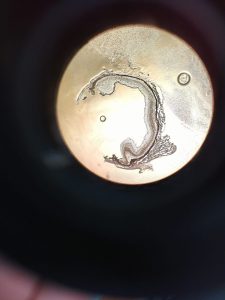
🔬 In the process, thanks to Oksana Kutsyr and Miguel Flores-Bellver, I learned that an experiment is not the image you see on a paper, but the six months spent growing organoids, the endless hours spent in the cell culture room, which are only rewarded when you see the perfection of your first baby in the confocal microscope. In the process, I gained another family and new friends who were there for me when I felt homesick.
🙏 For all this, I must thank Retorna_EU programme, my PI Javier Sancho and Universidad Católica de Valencia San Vicente Mártir, which gave me the opportunity to live this incredible experience. ”
- Maria from Telethon Institute is still in Alicante, where she is working together with Enola and Ceren and the rest of the lab team lead by prof. Nicolás Cuenca and prof. Natalia Martínez Gil.
- Claire from UCC finished a secondment experience at Salutem Insights. The secondment was really enriching, as Claire could have an insight from the industry’s perspective. For the next year Claire and her PI will value the possibility of doing a second part of the secondment. Here below her opinion on the weeks spent with the Salutem Insights team:
“For my first long secondment as a Retorna_EU fellow, I was fortunate enough to be hosted by Salutem Insights Ltd., whose mission is to advise companies seeking expertise in Irish market access and health economics.
👥 Right from the get-go I was given the opportunity to be directly involved in one of the team’s key projects and benefited tremendously from their openness and willingness to share their knowledge with me.
😍 Moreover, the enthusiasm and dedication of the Salutem team towards their work inspired me to continue on my PhD journey with further confidence.
🙌👏 Thank you Salutem!””
🌟Publications
During the month of November 2025 two publications have been published. Three of our PhD students have been involved:
1.The role of miRNAs in retinal physiology and in Inherited Retinal Disorders
Paola Brandi, Maria Zawadzka, Sandro Banfi
PMID: 41213174 DOI: 10.1152/physiol.00035.2025
PMID: 41270025 PMCID: PMC12637958 DOI: 10.1371/journal.pone.0335434
All publications are also available in the Publications section of the website and in the Zenodo RETORNA community.
🌟Radboudumc staff involved in 2 activities during November
*Promoting science during the Science day
On the 12th of November 2025 the Department of Ophthamology open its doors to all researchers and administrative staff with the aim to present the different research lines, groups and projects to the rest of the research community and administrative staff.
During the day around 40 participants learnt more about the research developped at the Department of Ophtamology. Our DC2 Yao presented her research, answered questions related to her project and invited participants to have a closer look at her research. during the entire day. She invited the participants to see how she and her team culture cells and retinal organoids in the laboratory.
*Dutch Ophthalmology PhD Students networking event
The DOPS conference (Dutch Ophthalmology PhD Students) is a yearly two-day conference and includes key note lectures, poster presentations and social activities for PhD students in the field of ophthalmology.The 2025 edition of the annual DOPS conference focused on “Wellbeing for YOU and EYE”.

On 21–22 November 2025, DOPS 2025 was held in Nijmegen and brought together eye care PhD candidates and junior researchers for two days of inspiration, learning,networking and collaboration.
Every year, a different team of PhD students is responsible for organizing this event. We are very glad to inform you that this year our DC Yao was part of the organization committee, acquiring experience in organizing scientific events, solving issues and working in team, among other skills that she acquired thanks to this experience.
Yao is “grateful for the opportunity to be part of the organizing team this year!! Helping organize DOPS was a valuable experience in teamwork and in supporting an event that brings PhD students together—while also providing a great opportunity to network with peers”.
In the name of RETORNA we thank DOPS conference for giving her this opportunity!
🌟 Boards rotation
RETORNA has 5 boards (managing, quality, research, training and communication). They work together to reach and fulfill the objectives of the project. DCs are also part of the 5 boards. In order to discover and participate in all the aspects of the project, DCs rotate every 6 months to another board. A new rotation period started in November 2025 and will go on until April 2026.
Greetings,
RETORNA team